Ernest Rutherford, a booming voiced Physicist once said “All science is either physics or stamp collecting.” He went on to win a Nobel Prize for...stamp collecting (chemistry). Chemistry has been called the central science, which is where physicists or biologists will claim a cool thing in chemistry as their own. So with that, let me share with you how physicists use the periodic table.
2019 is the International year of the periodic table, which is great for the chemists, but it’s also really good news for the physicists too. The periodic table is a phenomenally useful tool used by chemists and memorised by school students. It still makes mainstream media when a new element is found, and so it should.
The periodic table is more than just a smart list of well arranged elements used by chemists. physicists are just as excited about this year and what the periodic table means to us! so in 2019, I've been asking some physicists what their favourite element is and their answers have been very interesting.
Astronomy
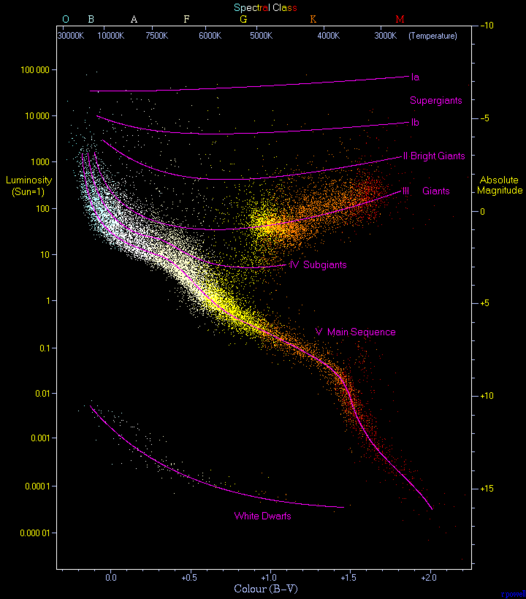
It’s a running joke in Astronomy that the universe is made of only three elements. Hydrogen (H, #1), Helium (He, #2) and the rest. Astronomers call these “heavy metals.” In some ways it is a bit silly and simplistic, but it also makes at least a bit of sense. The most abundant element in the universe is Hydrogen, followed by the second most abundant element, Helium. The first stars were Hydrogen and Helium, and stars are mostly made from these two elements. If you want to understand stars, you need to understand H and He.
Having said that, there are many astronomical processes that depend on other elements, like Lead (Pb, #82). A star is a machine for that “smashes” atomic nuclei together, and makes energy. It fuses elements together to make new elements. When that process can go no further that the stable element Lead, the process of nuclear fusion stops. This eliminates the outward pressure balancing the inward pull of gravity and it collapses, this is sometimes called a supernova, and it is spectacular. This event and other events like it, create unique and incredible conditions of energy and pressure that manage to make every other element in the periodic table. Incredible. You can’t have chemistry without Astronomy.
I asked Astronomer Professor, Tim Bedding what was his favourite element and he said Technetium (Tc, #43). Why? Because there are some processes that happen when a star enters a red giant phase (this is when it’s fusing mostly Helium, rather than Hydrogen) of its life, through transfers or neutrons in the star, it creates Technetium. So if we observe Technetium in a star, then we know that it is in its red giant phase. Professor Bedding's work is on Asteroseismology (starquakes), which is using information about stars to tell more about the exoplanets that orbit them.
Quantum physics
Quantum physics is a fascinating area of physics that we’re spending a lot of time thinking about. It spans the gap between people that change physics and physics that changes people. From fundamental understanding of the natural world, to applications that will change how we interact with the natural world. When dealing with interactions of matter at such small scales, we really need to understand properties of some elements and components of elements, such as electrons.
I asked quantum physics researcher Professor Michael Biercuk why the quantum control group uses Ytterbium (Yb, #70) and Beryllium (Be, #4) in their research into quantum control, why they use those two elements. His response was:
“Because you want atoms that when singly ionized have a single valence electrons like Hydrogen and you want atoms with optical transitions that are accessible with commercial lasers.”
In other words, electrons are really important in quantum physics and we need to be able to access them in a predictable way, using predictable technologies.
Superconductivity
There is an international race on at the moment to find the potentially revolutionary solutions to achieving room temperature superconductivity. Properties of superconductors include zero electrical resistance and perfect magnetism, ridiculously efficient computation, electricity storage & transport and revolutionary medical applications. One of the dirty little secrets in science is that we still don’t understand fully the physics of superconductivity. We do know a couple of things though:
Optics
Without fibre optics, you couldn’t download a movie – the data transfer rate would be too low across the network. Computers, communications, the internet and just about every part of our modern life depends on optics. The field of optics going through a very important period at the moment. We are pushing the limits of how much information we can send through an optical fibre. This is an issue as humans are increasing in number while also increasing the amount of information we’re sending to each other. For example there are about 300 hours of videos uploaded to YouTube every minute. To solve this problem of the data transfer rate being too low in fibre optic cables, we either need more higher capacity cables, at great expense, or develop techniques to use the existing cables more efficiently.
This is where group 16 of the periodic table which is the group that contains Oxygen (O, #8) comes in. This group, called the Chalcogens is the basis of a lot of research into more efficient fibre optics with some very cool properties. A specific type of Chalcogenide glass which is a mic of Arsenic (As, #33) and Sulfur (S, #16) As2S3, has some pretty fantastic properties, like slowing light down and changing the frequency of light. Essentially, researchers are trying to do all the things that we can do with electrons and wires, but with light.
I asked optical physicist Professor Martijn De Sterke what his favourite element is and he said it was Osmium (Os, #76):
Because of its density and he thought it would be cool to hold it in your hands. It is the element with the highest density (22 tons per m3), coming in at twice the density of lead. I’d love to feel it in my hands.
Medical Physics
Medical physicists use the properties of a few elements such as Iodine (I, #53) and Strontium (Sr, #38) in order to create radiation for diagnosis and treatment of some conditions and diseases. For example, we’ll use beta emitters such as Strontium and fire them at a high density material like Lead (Pb, # 82). When we do that we create x-rays through a process called Bremsstrahlung Radiation, or braking radiation. Literally, slowing down electrons produces x-rays.
I asked Medical Physicist Professor Zdenka Kuncic and she said her favourite element was Technetium (Tc, #43) as it is used in medical physics research a lot as a tracer element which when injected into a patient's bloodstream helps with the imaging of some parts of the body. In addition, the half life of Technetium is very short so it doesn’t stay around in the body too long, so it’s relatively safe.
Let’s all party!
The periodic table of the elements is one of the most recognisable and useful tools in science, and while we should be celebrating the chemists, as a physicist I’d really like to join in the party with the chemists and all scientists. It’s great to have a periodic reminder that scientists are collaborative and welcoming and love to share successes and knowledge. After all, that is in fact how science works.
If you’re wondering, my favourite element is Ruthenium (Ru, #44). My daughters favourite number is 44 and Ruthenium is element number 44. It’s atomic mass is 101.7, and my house number is 101. Also, my wife’s name is Ruth!
What’s your favourite element?
2019 is the International year of the periodic table, which is great for the chemists, but it’s also really good news for the physicists too. The periodic table is a phenomenally useful tool used by chemists and memorised by school students. It still makes mainstream media when a new element is found, and so it should.
The periodic table is more than just a smart list of well arranged elements used by chemists. physicists are just as excited about this year and what the periodic table means to us! so in 2019, I've been asking some physicists what their favourite element is and their answers have been very interesting.
Astronomy
It’s a running joke in Astronomy that the universe is made of only three elements. Hydrogen (H, #1), Helium (He, #2) and the rest. Astronomers call these “heavy metals.” In some ways it is a bit silly and simplistic, but it also makes at least a bit of sense. The most abundant element in the universe is Hydrogen, followed by the second most abundant element, Helium. The first stars were Hydrogen and Helium, and stars are mostly made from these two elements. If you want to understand stars, you need to understand H and He.
 |
| Image: https://i.stack.imgur.com/W144r.png |
Having said that, there are many astronomical processes that depend on other elements, like Lead (Pb, #82). A star is a machine for that “smashes” atomic nuclei together, and makes energy. It fuses elements together to make new elements. When that process can go no further that the stable element Lead, the process of nuclear fusion stops. This eliminates the outward pressure balancing the inward pull of gravity and it collapses, this is sometimes called a supernova, and it is spectacular. This event and other events like it, create unique and incredible conditions of energy and pressure that manage to make every other element in the periodic table. Incredible. You can’t have chemistry without Astronomy.
I asked Astronomer Professor, Tim Bedding what was his favourite element and he said Technetium (Tc, #43). Why? Because there are some processes that happen when a star enters a red giant phase (this is when it’s fusing mostly Helium, rather than Hydrogen) of its life, through transfers or neutrons in the star, it creates Technetium. So if we observe Technetium in a star, then we know that it is in its red giant phase. Professor Bedding's work is on Asteroseismology (starquakes), which is using information about stars to tell more about the exoplanets that orbit them.
Quantum physics
Quantum physics is a fascinating area of physics that we’re spending a lot of time thinking about. It spans the gap between people that change physics and physics that changes people. From fundamental understanding of the natural world, to applications that will change how we interact with the natural world. When dealing with interactions of matter at such small scales, we really need to understand properties of some elements and components of elements, such as electrons.
I asked quantum physics researcher Professor Michael Biercuk why the quantum control group uses Ytterbium (Yb, #70) and Beryllium (Be, #4) in their research into quantum control, why they use those two elements. His response was:
“Because you want atoms that when singly ionized have a single valence electrons like Hydrogen and you want atoms with optical transitions that are accessible with commercial lasers.”
In other words, electrons are really important in quantum physics and we need to be able to access them in a predictable way, using predictable technologies.
Superconductivity
 |
| Image: Tom Gordon |
- At extremely low temperatures, metals like Copper (Cu, #29) Niobium (Nb, #41) and Mercury (Hg, #80) are particularly useful superconductors.
- At slightly higher temperatures, ceramics like Yttrium Barium Copper Oxide, YBa2Cu3O7, (Y, #39, Ba, #56, Cu, #29, O, #8) seem to work, but we aren’t exactly sure why. Most other ceramic superconductors seem to have Copper Oxides as their base. It’s got something to do with the structure of the crystals, but we’re not 100% sure
- An exciting avenue for discovering new superconductors that operate at room temperatures, rather than at cryogenic temperatures is certainly in the structure of the materials we’re looking into. Perovskite, or calcium titanate (CaTiO3) (Ca, #20, Ti, #22, O, #8) is an interesting material that some people are getting very excited about.
Optics
Without fibre optics, you couldn’t download a movie – the data transfer rate would be too low across the network. Computers, communications, the internet and just about every part of our modern life depends on optics. The field of optics going through a very important period at the moment. We are pushing the limits of how much information we can send through an optical fibre. This is an issue as humans are increasing in number while also increasing the amount of information we’re sending to each other. For example there are about 300 hours of videos uploaded to YouTube every minute. To solve this problem of the data transfer rate being too low in fibre optic cables, we either need more higher capacity cables, at great expense, or develop techniques to use the existing cables more efficiently.
This is where group 16 of the periodic table which is the group that contains Oxygen (O, #8) comes in. This group, called the Chalcogens is the basis of a lot of research into more efficient fibre optics with some very cool properties. A specific type of Chalcogenide glass which is a mic of Arsenic (As, #33) and Sulfur (S, #16) As2S3, has some pretty fantastic properties, like slowing light down and changing the frequency of light. Essentially, researchers are trying to do all the things that we can do with electrons and wires, but with light.
 |
| Image: Wikipedia |
I asked optical physicist Professor Martijn De Sterke what his favourite element is and he said it was Osmium (Os, #76):
Because of its density and he thought it would be cool to hold it in your hands. It is the element with the highest density (22 tons per m3), coming in at twice the density of lead. I’d love to feel it in my hands.
Medical Physics
Medical physicists use the properties of a few elements such as Iodine (I, #53) and Strontium (Sr, #38) in order to create radiation for diagnosis and treatment of some conditions and diseases. For example, we’ll use beta emitters such as Strontium and fire them at a high density material like Lead (Pb, # 82). When we do that we create x-rays through a process called Bremsstrahlung Radiation, or braking radiation. Literally, slowing down electrons produces x-rays.
I asked Medical Physicist Professor Zdenka Kuncic and she said her favourite element was Technetium (Tc, #43) as it is used in medical physics research a lot as a tracer element which when injected into a patient's bloodstream helps with the imaging of some parts of the body. In addition, the half life of Technetium is very short so it doesn’t stay around in the body too long, so it’s relatively safe.
Let’s all party!
The periodic table of the elements is one of the most recognisable and useful tools in science, and while we should be celebrating the chemists, as a physicist I’d really like to join in the party with the chemists and all scientists. It’s great to have a periodic reminder that scientists are collaborative and welcoming and love to share successes and knowledge. After all, that is in fact how science works.
 |
| Image: Wikipedia |
What’s your favourite element?